Вода | 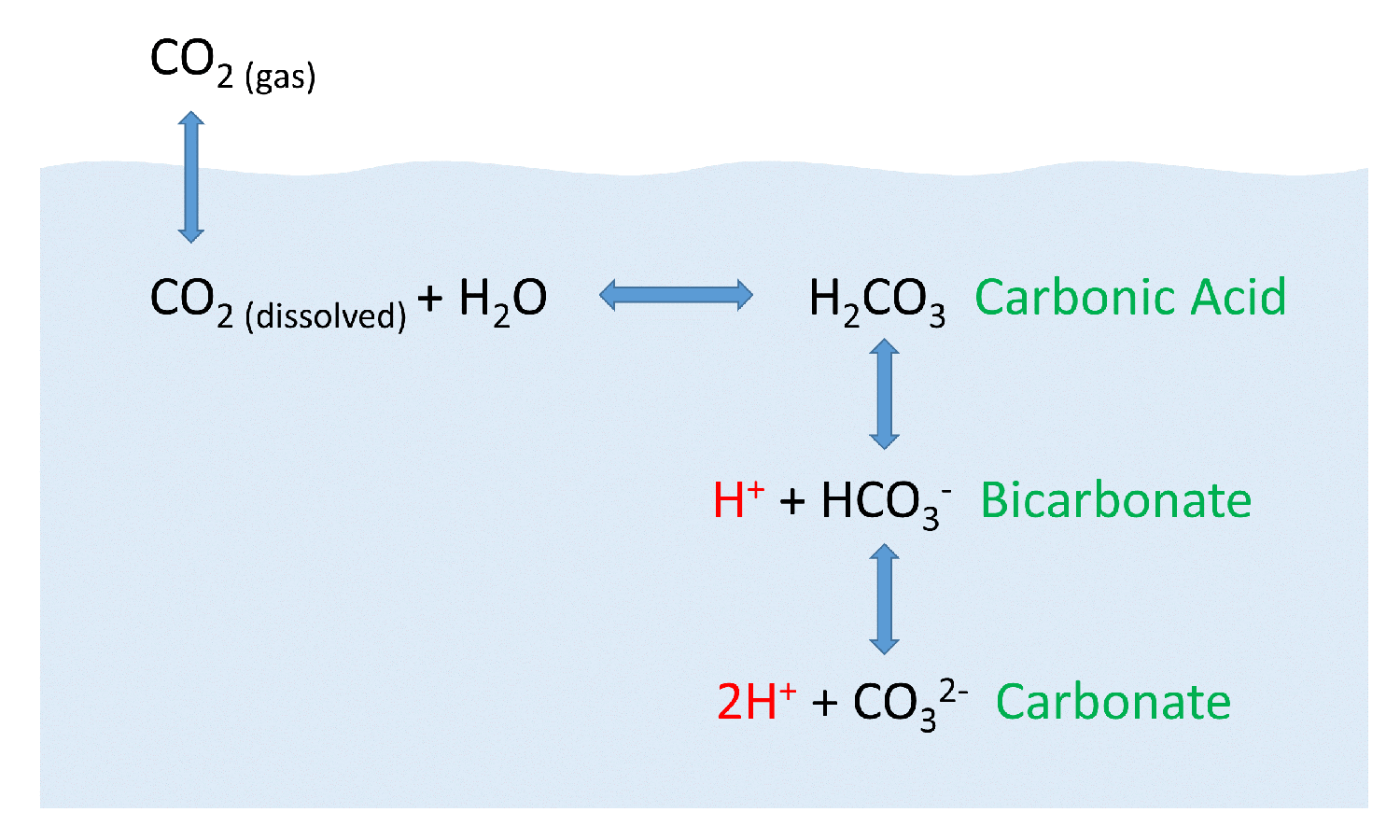 |
28 мая 2020 г. · The amount of CO2 dissolved in water is proportional to the outer pressure. At 20°C, 1 liter water dissolves about 1.7 g CO2 at normal pressure ... acid base - How does CO2 'dissolve' in water (or blood)? If CO2 is nonpolar how come much more dissolves in water ... Другие результаты с сайта chemistry.stackexchange.com |
30 июл. 2024 г. · CO2 is soluble because water molecules are attracted to these polar areas. The bond between carbon and oxygen is not as polar as the bond ... |
Carbon dioxide content in air is only 0.03%, but it is highly soluble in water unlike oxygen and one volume of CO 2 dissolves in equal volume of water. |
CO2 can exist solvated in water as CO2 but some (most) molecules will actually react with a water molecule to form H2CO3 which, in solution, will dissociate ... |
23 авг. 2020 г. · CO2 is non polar, but when it dissolves it react with a water molecule to produce H2CO3 and some HCO3(-) both of which are polar. |
24 сент. 2018 г. · Carbon dioxide (CO2) is slightly soluble in water under ambient conditions. At room temperature CO2 will dissolve to a concentration of about ... |
When CO2 dissolves in water, it forms a weak acid, which can affect the geochemistry of subsurface rocks as well marine organisms synthesizing shells. A method ... |
The highest solubility of CO2 in water was 1.320 gr/L. On the other hand, the pH values of CO2 in water decreased with increasing of CO2 gas pressure. |
27 июл. 2023 г. · First, CO2 is not very soluble in water at atmospheric pressure. Second, some of the vibrational states of CO2 do develop an intermittent ... |
Запросы по теме
| Novbeti > |
| Axtarisha Qayit Anarim.Az Anarim.Az Sayt Rehberliyi ile Elaqe Saytdan Istifade Qaydalari Anarim.Az 2004-2023 |